This posting focuses on one specific type of artificial pancreas (AP), called a bihormonal artificial pancreas, or sometimes a "bionic pancreas". As with all artificial pancreas discussion, some people don't think these devices are a cure (they are just treatment methods), while others do consider them a cure.
Many months ago, I attended a CarbDM event, where Dr. Ed Damiano discussed recent testing of bihormonal artificial pancreas. (CarbDM is a support group for families with type-1 diabetes, which operates in several cities in Northern California, including San Francisco, San Jose, and Sacramento. If you live there, you should see what they're doing:
http://carbdm.org/.) Anyway, as soon as I heard the presentation, and the great results, I knew I needed to blog on it. But there was never time, and it got pushed out, and now it's six months later. My bad.
The good news is that diaTribe has published really good summaries of Dr. Damiano's work, so I encourage you to read their postings now, and then come back to finish reading this posting:
(Brief digression on diaTribe
www.diatribe.org: It is a free news service covering diabetes (both type-1 and type-2) which was spun off from Close Concerns, which is a commercial news service covering diabetes. DiaTribe is a great source of news, because they are covering in depth something they understand very well. They do their own analysis, so they are not just mindlessly reprinting other people's press releases, which is common from other news sources.)
Now, back to the bihormonal artificial pancreas: The bihormonal artificial pancreas delivered a level of control (without human intervention) good enough to be called a cure. The estimated A1c numbers were 6.2 in one study and 6.6 in the other. The average BG numbers were 133 and 142. At the same time, the number of BG measures below 60 was lower when using the bihormonal artificial pancreas. So they had better control and fewer lows.
What is a Bi-hormonal Artificial Pancreas?
Artificial Pancreas refers to a pump / continuous blood glucose monitor / computer combination that controls blood sugar automatically based on data from the monitor. All of these are existing technologies, but used together in a new way. It is also called the "closed loop". Bi-hormonal refers to using both insulin and glucagon. So a bi-hormonal AP can give both insulin and glucagon, based on blood sugar levels, to automatically keep a person's blood sugar levels in range, using existing hardware technology.
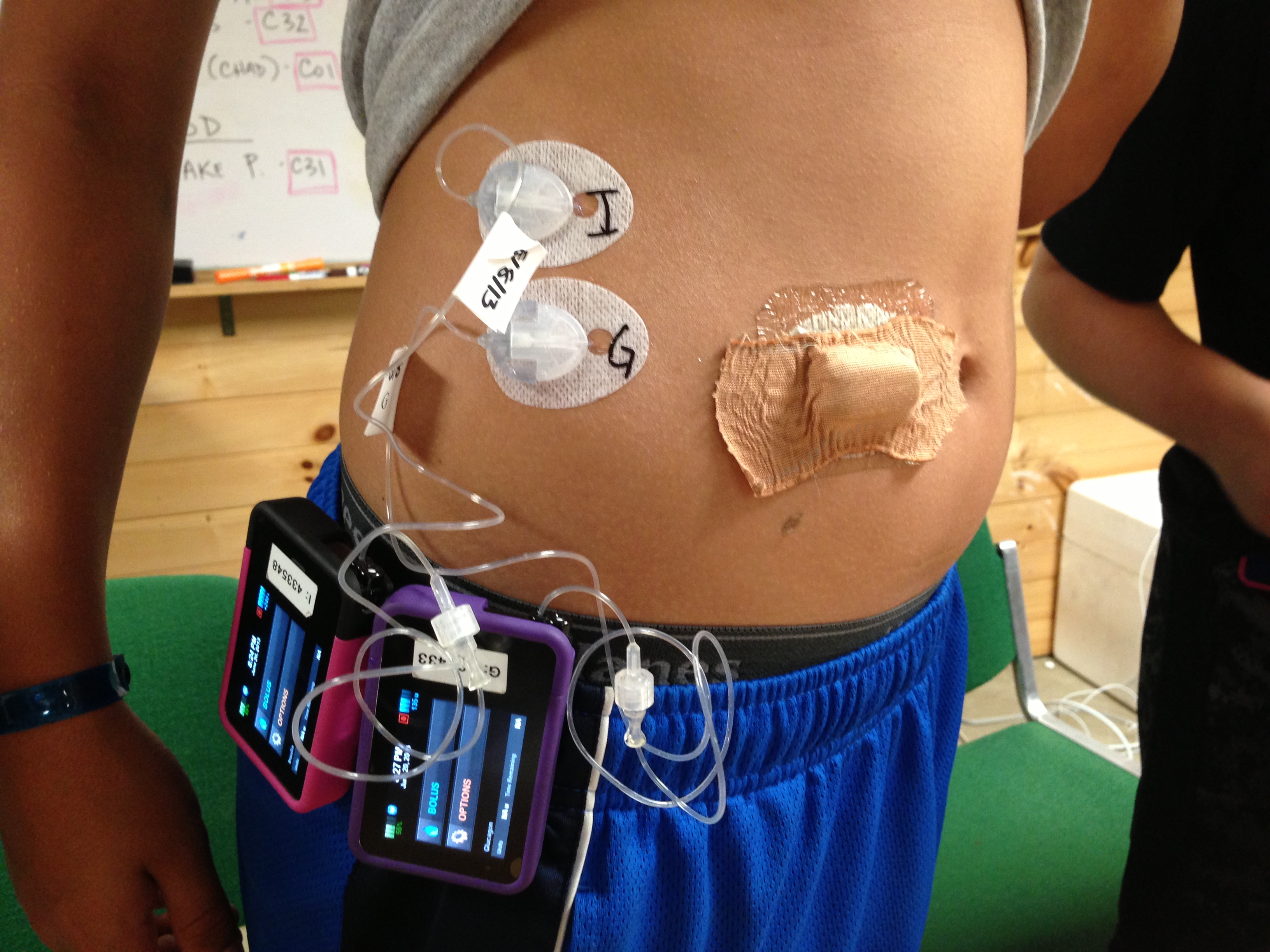
The current bi-hormonal AP is composed of a G4 CGM, two T:slim pumps, and iPhone hardware that has had the phone software removed and replaced by software to control the pumps. The current experimental prototype sounds "clunky" in the extreme. Basically, you are wearing two pumps and a CGM device. There are three separate sets, etc.
Take a look at this photo (hosted on diaTribe's site), to see just how "clunky" and crowded it is:
A "classic" AP is insulin only, so it can lower blood glucose, but not raise it. A person eats sugar to raise their BG levels, but the AP doesn't control that. A bi-hormonal AP can both raise and lower a person's BG levels, so it can have much better control.
As I said before, the current blood glucose control, as seen in already completed clinical trials, is spectacular. In my opinion, it is good enough for a cure right now, even if they don't improve it at all moving forward.
What's the Plan Moving Forward?
The plan is to do one large, multiple site clinical trial on adults who work in hospitals and have type-1 diabetes. The goal of this study is to have people living their normal lives, including going to work, and using the bihormonal AP, and yet be close to medical help, for trial safety. I think of this as being a large phase-II trial. This trial will use the current "prototype" hardware.
Next, the researchers will develop the real hardware: the hardware they expect to sell, and use that to run two large phase-III ("pivotal") trials.
The lead researcher, Dr. Ed Damiano, is totally committed to getting this device on the market by 2017.
Discussion
So the big question is, will it be on the market by 2017? And the answer is "no one knows". But nobody likes that answer, so there is rampant speculation, starting right here. In my opinion, there are several risks to the 2017 date:
- Glucagon. A bi-hormonal pump needs glucagon which can stay in the pump for 3 days, and longer would be better. Current glucagon is not stable for long periods of time, which is why you mix a powder with water just before injecting it. People in the current studies had to refill their glucagon every day, so that it was fresh and active when used. That's ok for testing, but not in real use. There are two mitigation strategies, for this risk: First there are two companies who are trying to get FDA approval for long lasting Glucagon right now. If either one gets approval before 2017, the problem is solved. Second, even if this problem is not solved at all, early users will need to refill glucagon each day, which is a hassle, but can be done.
- FDA Approval. For APs this has not been predictable. The Medtronic Veo took 31 months, after it had been approved in Europe! However, the phase-II trials show a level of BG control much better than existing pumps or insulin injections. I would expect the phase-III trials to be even better, and therefore there will be strong data to help the FDA grant approval. Plus (as with the Veo) I assume that patient advocacy organizations, such as JDRF, will be willing to launch a publicity program to "help" the FDA move forward.
- Business Issues. The bihormonal AP will need a company behind it, to become reality. Type-1 diabetics are not going to buy a research project, and insurance will not pay for one. So there must be a commercial company that builds, markets, and sells these things. There are several ways this could happen: they could create a new company to build and sell a bi-hormonal artificial pancreas, or they could form a company which owns the intellectual property (patents and fabrication know-how). That company would then either licence an existing pump manufacturer to produce bihormonal APs, or sell itself to an existing pump company, which would then own the necessary patents to make bihormonal APs. In any case there is risk, but it is the "normal" risk of commercial development, not the extra risk of scientific research.
- Engineering Issues. This bi-hormonal pump is a integration of several different components: two pumps, two hormones, a computer algorithm, a controller, a CGM, etc. None of this is really new technology, but the current schedule assume that putting it all together will happen quickly and without a serious issue cropping up. But there is always the risk of one of these issues cropping up, at the worst possible time.
But Is It A Cure?
I think that each of us has to decide for themselves what is a cure, and what is not. So I'm not going to tell you this is a cure, or this is not. (I am going to ask that you not write me nasty emails saying I'm an shill for even talking about APs as a possible cure: just because you don't think they are a cure, doesn't mean that everyone agrees with you. All kinds of different people read this blog.)
When I talk to people, most of them accept an implanted (internal) AP as a cure for type-1, however if you take that same functionality and carry it outside the body, then most people do not consider it a cure.
The bi-hormonal prototype (ie. the thing they are testing now) requires the user to check blood glucose only to calibrate the CGM. It requests that the user tell it about meals, but does not require any carb counting (the user clicks a button to say breakfast, lunch, or dinner). The user does need to refill the insulin every 3 days, and during testing, refill the glucagon every day. However, the researchers expect long lasting glucagon to be available as part of the commercial product.
In testing done so far, about 2/3s of the meals were actually warned by the user. In the other 1/3 of the cases, the user forgot or didn't bother. So the numbers above include failure to warn 1/3 of the time. The researcher estimate that if the users never warned of a meal, that their average BG numbers would rise about 10, and the average A1C would rise about 0.1.
Abstracts on papers for the bihormonal artificial pancreas:
http://www.ncbi.nlm.nih.gov/pubmed/24224750
http://www.ncbi.nlm.nih.gov/pubmed/23602044
Here is a link to diaTribe coverage long storage Glucagon:
http://diatribe.org/issues/59/new-now-next/4#!
Clinical Trial Records for these studies:
http://clinicaltrials.gov/ct2/show/NCT00811317
http://clinicaltrials.gov/ct2/show/NCT01161862
http://clinicaltrials.gov/ct2/show/NCT01762059
http://clinicaltrials.gov/ct2/show/NCT01833988
Let me leave you with a diagram showing what happened to a group of people on the bihormonal artificial pancreas. Notice that every single person ended up with an average BG between about 100 and 150. My slogan for this diagram is "no diabetic left behind". Even the guy who started out averaging over 220, ended up in range:
(Note: this diagram comes from a children-with-diabetes page which is collecting tax deductable donations for this research:
http://cwdfoundation.org/BionicPancreas.html.)
Other Bi-hormonal Artificial Pancreas Projects
The only other research that I know of into bihormonal APs is being done in Europe, and is called the "PCDIAB" project. You can read about it here:
http://pcdiab.eu/
and see a related clinical trial record here:
http://clinicaltrials.gov/ct2/show/NCT01916265
Joshua Levy
http://cureresearch4type1diabetes.blogspot.com
publicjoshualevy at gmail dot com
All the views expressed here are those of Joshua Levy, and nothing here is official JDRF, JDCA, or Tidepool news, views, policies or opinions. My daughter has type-1 diabetes and participates in clinical trials, which might be discussed here. My blog contains a more complete non-conflict of interest statement. Thanks to everyone who helps with the blog.