Many months ago, I attended a CarbDM event, where Dr. Ed Damiano discussed recent testing of bihormonal artificial pancreas. (CarbDM is a support group for families with type-1 diabetes, which operates in several cities in Northern California, including San Francisco, San Jose, and Sacramento. If you live there, you should see what they're doing: http://carbdm.org/.) Anyway, as soon as I heard the presentation, and the great results, I knew I needed to blog on it. But there was never time, and it got pushed out, and now it's six months later. My bad.
The good news is that diaTribe has published really good summaries of Dr. Damiano's work, so I encourage you to read their postings now, and then come back to finish reading this posting:
(Brief digression on diaTribe www.diatribe.org: It is a free news service covering diabetes (both type-1 and type-2) which was spun off from Close Concerns, which is a commercial news service covering diabetes. DiaTribe is a great source of news, because they are covering in depth something they understand very well. They do their own analysis, so they are not just mindlessly reprinting other people's press releases, which is common from other news sources.)
What is a Bi-hormonal Artificial Pancreas?
Artificial Pancreas refers to a pump / continuous blood glucose monitor / computer combination that controls blood sugar automatically based on data from the monitor. All of these are existing technologies, but used together in a new way. It is also called the "closed loop". Bi-hormonal refers to using both insulin and glucagon. So a bi-hormonal AP can give both insulin and glucagon, based on blood sugar levels, to automatically keep a person's blood sugar levels in range, using existing hardware technology.
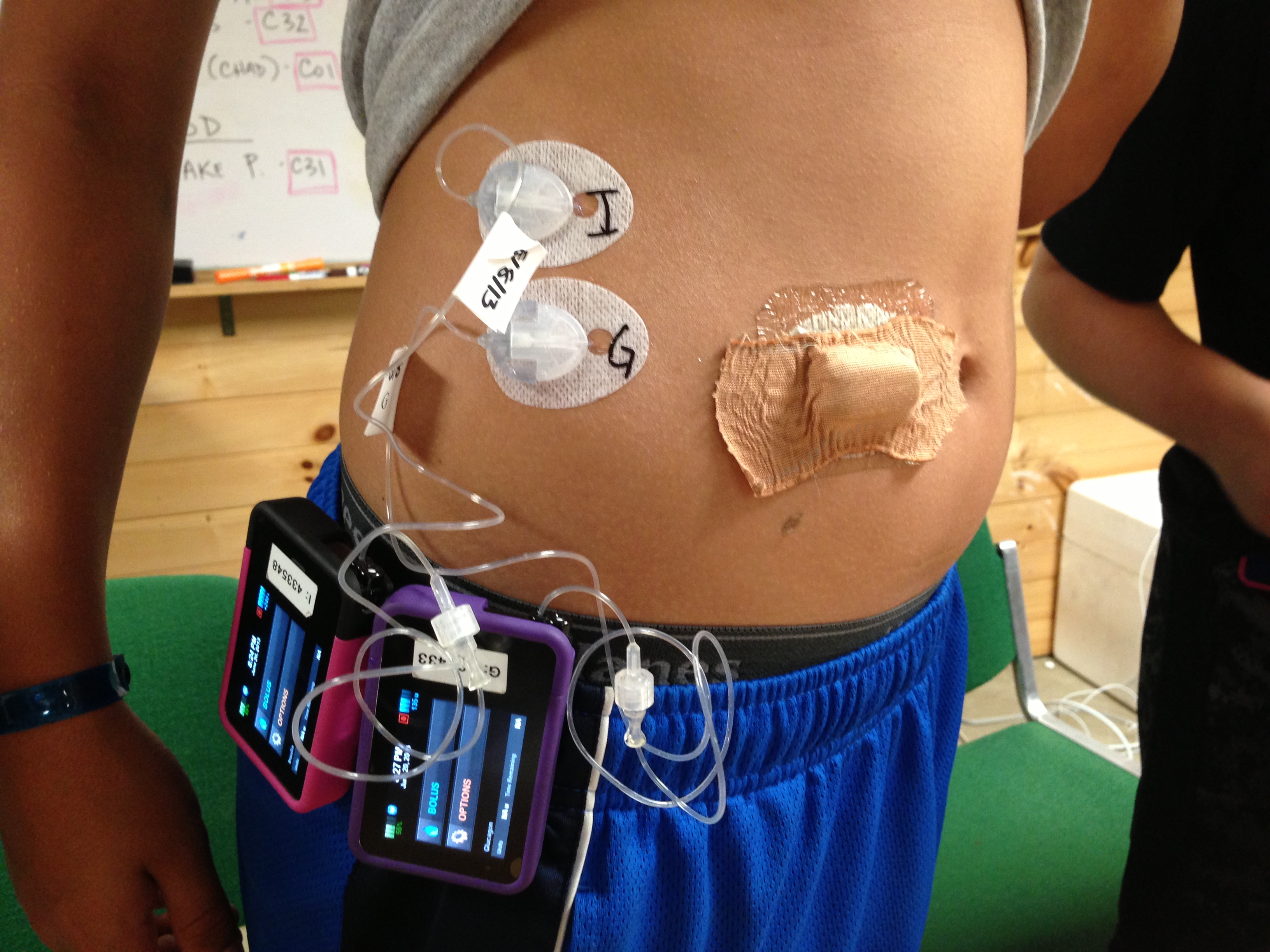
The current bi-hormonal AP is composed of a G4 CGM, two T:slim pumps, and iPhone hardware that has had the phone software removed and replaced by software to control the pumps. The current experimental prototype sounds "clunky" in the extreme. Basically, you are wearing two pumps and a CGM device. There are three separate sets, etc.
Take a look at this photo (hosted on diaTribe's site), to see just how "clunky" and crowded it is:
A "classic" AP is insulin only, so it can lower blood glucose, but not raise it. A person eats sugar to raise their BG levels, but the AP doesn't control that. A bi-hormonal AP can both raise and lower a person's BG levels, so it can have much better control.
As I said before, the current blood glucose control, as seen in already completed clinical trials, is spectacular. In my opinion, it is good enough for a cure right now, even if they don't improve it at all moving forward.
What's the Plan Moving Forward?
The plan is to do one large, multiple site clinical trial on adults who work in hospitals and have type-1 diabetes. The goal of this study is to have people living their normal lives, including going to work, and using the bihormonal AP, and yet be close to medical help, for trial safety. I think of this as being a large phase-II trial. This trial will use the current "prototype" hardware.
Next, the researchers will develop the real hardware: the hardware they expect to sell, and use that to run two large phase-III ("pivotal") trials.
The lead researcher, Dr. Ed Damiano, is totally committed to getting this device on the market by 2017.
Discussion
So the big question is, will it be on the market by 2017? And the answer is "no one knows". But nobody likes that answer, so there is rampant speculation, starting right here. In my opinion, there are several risks to the 2017 date:
- Glucagon. A bi-hormonal pump needs glucagon which can stay in the pump for 3 days, and longer would be better. Current glucagon is not stable for long periods of time, which is why you mix a powder with water just before injecting it. People in the current studies had to refill their glucagon every day, so that it was fresh and active when used. That's ok for testing, but not in real use. There are two mitigation strategies, for this risk: First there are two companies who are trying to get FDA approval for long lasting Glucagon right now. If either one gets approval before 2017, the problem is solved. Second, even if this problem is not solved at all, early users will need to refill glucagon each day, which is a hassle, but can be done.
- FDA Approval. For APs this has not been predictable. The Medtronic Veo took 31 months, after it had been approved in Europe! However, the phase-II trials show a level of BG control much better than existing pumps or insulin injections. I would expect the phase-III trials to be even better, and therefore there will be strong data to help the FDA grant approval. Plus (as with the Veo) I assume that patient advocacy organizations, such as JDRF, will be willing to launch a publicity program to "help" the FDA move forward.
- Business Issues. The bihormonal AP will need a company behind it, to become reality. Type-1 diabetics are not going to buy a research project, and insurance will not pay for one. So there must be a commercial company that builds, markets, and sells these things. There are several ways this could happen: they could create a new company to build and sell a bi-hormonal artificial pancreas, or they could form a company which owns the intellectual property (patents and fabrication know-how). That company would then either licence an existing pump manufacturer to produce bihormonal APs, or sell itself to an existing pump company, which would then own the necessary patents to make bihormonal APs. In any case there is risk, but it is the "normal" risk of commercial development, not the extra risk of scientific research.
- Engineering Issues. This bi-hormonal pump is a integration of several different components: two pumps, two hormones, a computer algorithm, a controller, a CGM, etc. None of this is really new technology, but the current schedule assume that putting it all together will happen quickly and without a serious issue cropping up. But there is always the risk of one of these issues cropping up, at the worst possible time.
But Is It A Cure?
I think that each of us has to decide for themselves what is a cure, and what is not. So I'm not going to tell you this is a cure, or this is not. (I am going to ask that you not write me nasty emails saying I'm an shill for even talking about APs as a possible cure: just because you don't think they are a cure, doesn't mean that everyone agrees with you. All kinds of different people read this blog.)
When I talk to people, most of them accept an implanted (internal) AP as a cure for type-1, however if you take that same functionality and carry it outside the body, then most people do not consider it a cure.
The bi-hormonal prototype (ie. the thing they are testing now) requires the user to check blood glucose only to calibrate the CGM. It requests that the user tell it about meals, but does not require any carb counting (the user clicks a button to say breakfast, lunch, or dinner). The user does need to refill the insulin every 3 days, and during testing, refill the glucagon every day. However, the researchers expect long lasting glucagon to be available as part of the commercial product.
In testing done so far, about 2/3s of the meals were actually warned by the user. In the other 1/3 of the cases, the user forgot or didn't bother. So the numbers above include failure to warn 1/3 of the time. The researcher estimate that if the users never warned of a meal, that their average BG numbers would rise about 10, and the average A1C would rise about 0.1.
Abstracts on papers for the bihormonal artificial pancreas:
http://www.ncbi.nlm.nih.gov/pubmed/24224750
http://www.ncbi.nlm.nih.gov/pubmed/23602044
Here is a link to diaTribe coverage long storage Glucagon:
http://diatribe.org/issues/59/new-now-next/4#!
Clinical Trial Records for these studies:
http://clinicaltrials.gov/ct2/show/NCT00811317
http://clinicaltrials.gov/ct2/show/NCT01161862
http://clinicaltrials.gov/ct2/show/NCT01762059
http://clinicaltrials.gov/ct2/show/NCT01833988
Let me leave you with a diagram showing what happened to a group of people on the bihormonal artificial pancreas. Notice that every single person ended up with an average BG between about 100 and 150. My slogan for this diagram is "no diabetic left behind". Even the guy who started out averaging over 220, ended up in range:
(Note: this diagram comes from a children-with-diabetes page which is collecting tax deductable donations for this research: http://cwdfoundation.org/BionicPancreas.html.)
Other Bi-hormonal Artificial Pancreas Projects
The only other research that I know of into bihormonal APs is being done in Europe, and is called the "PCDIAB" project. You can read about it here:
http://pcdiab.eu/
and see a related clinical trial record here:
http://clinicaltrials.gov/ct2/show/NCT01916265
Joshua Levy
http://cureresearch4type1diabetes.blogspot.com
publicjoshualevy at gmail dot com
All the views expressed here are those of Joshua Levy, and nothing here is official JDRF, JDCA, or Tidepool news, views, policies or opinions. My daughter has type-1 diabetes and participates in clinical trials, which might be discussed here. My blog contains a more complete non-conflict of interest statement. Thanks to everyone who helps with the blog.
9 comments:
Fantastic summary on the bionic pancreas. Been T1D for 18 years and in the last year have found a way to dramatically lower my A1c, but I am still so excited for this technology because it would finally take away so much of the work.
Will everyone be able to afford it. The cost of consumables can be out of the reach for most people. I personally feel enough people are not paying attention to encapsulation. Which will require NO human intervention. No refills or anything.
Sorry meant to say also thanks for your work as always Josh.
This is my "stock answer" whenever anyone asks about the cost of cures:
http://cureresearch4type1diabetes.blogspot.com/2011/11/future-cost-of-type-1-cures.html
Although the cost of a Bihormonal artificial pancreas is likely to higher than the cost of a pump and the cost of a CGM. Basically, it will be those two, plus the glucagon, at a minimum.
As for comparisons to encapsulation, if you look at actual BG control as shown today in clinical trials, BhAP is much farther along than any encapsulation being tested. Of course, there are many other comparisons that might be made, that might be more important.
I just read your third bullet point about technology and there was just a whole discussion on LinkedIn JDRF board site in which I said the same thing. But however pumps still cost well over 5,000 dollars and other diabetes technology seem to go up not down. The reason being is there is lot less competition in the diabetes technology device field. But information technology and in computers there is a whole lot competition. So I see the cost of technology "cures" being rather expensive because of this lack of competition. That of course is just my take on the matter which could be wrong :-) One last thing I truly believe Viacyte is into something this is why they are not saying much. :-)
Hi Joshua,
Firstly, I must say I love your blog. I am currently writing an essay about LADA for my nursing degree and your blog makes it nice and easy to find the links to all the relevant research for my future treatment discussion. :)
I disagree with your estimated cost and remarks about "clunkiness" about the finished product. The Animas Vibe has an in-built Dexcom 4 and is essentially a pump combined with a CGM. If you have ever taken a mobile phone apart, you know that the 'heart' of it is a minute processor; but at the end of the day, each pump already has one. I am sure that the final product would hardly be larger than an older style pump and the cost would be not much more than a pump.
I think the issues around the AP lie in the need for several injection/testing sites (much higher cost for consumables) and the need to recalibrate the CGM regularly. The latter is largely due to what the CGM is actually measuring. I personally believe that the current CGM technology is not advanced enough to make the AP work better than an insulin pump because you will still rely heavily on BGL measurements. And the price of CGM sensors is so horrendous that many govts and insurance companies won't pay for it. This unfortunately means that an independent AP producing company is out of the picture; and I personally believe that it is more likely that the technology will be picked up by pump companies.
As with any pump technology, there are non-pharmaceutical consumables required. The associated tubing etc... is usually very expensive. If you need to buy insulin/glucagon (directly or through insurance) and you need tubing, disposable cgm connections, etc... then you are being continuously treated (at a great expense). You are not cured. It isn't a cure if you get sick when you stop treating yourself. To say that this a cure is to say that AZT is a cure to AIDS. Only a salesman would try to have you believe that (and this is what I really think this study is about... making money). This is merely a treatment with dubious prospects of convenience with potentially 2 to 3 times the cost of current pump technology with pump makers raking in continuous profit on overpriced consumables.
Unistem Biosciences's mission is to find a cure for type 1 diabetes and its complications through the support of research. caused by the body's own immune system attacking and destroying insulin-making beta cells.
I'm reviewing Unistem right now, but at the moment, I can not find any evidence that they have ever cured (or even improved) anyone's type-1 diabetes). I don't see any papers, or any reporting of results for groups of people.
If you've got any evidence that Unistem helps type-1 diabetics, please email it to me at publicjoshualevy at g mail dot com. Otherwise, I'll remove your comment.
Post a Comment