Ladarixin is a drug being develop by Dompé (an Italian company). It inhibits activity on parts of the immune system called the IL-8 receptor (which has two subtypes: IL-8a and IL-8b). Dompé hopes that this will stop the progression of type-1 diabetes.
Before I discuss the phase-III study, I want to say something very important: A previous phase-II study, done by the same company (Dompé) of the same drug (Ladarixin) was successful when measured at 6 months, but unsuccessful when measured at 12 months (and 12 months was the primary end point). I discuss previous results below. However, the previous study gave people about 3 months of treatment, but the new study gives them about 12 months, a much longer treatment protocol.
The Phase-III Study
This is a big study. Phase-III studies for T1D are usually about 300 people, and this one is 327. Also, 2/3s of the people in the study will get the treatment; only 1/3 are controls who get the placebo. It started near the end of 2020, and they plan to finish in mid 2025.
This study will enroll honeymooners (within 180 days of diagnosis). About 200 will be children (aged between 14 and 17), and about 127 will be adults (between 18 and 45).
Ladarixin is a pill which people will take twice a day for two weeks, and then not take for two more weeks, and then they will repeat this cycle for a year. They will then be followed for an additional year. The primary results are C-peptide and A1c measurements after the year of treatment, and secondary results include these same measures throughout the 2 year trial and several more interesting outcomes. These include time in range, low blood glucose episodes, and patients not requiring injected insulin (!).
An interesting design point of this study is that it is fully blinded for the first 18 months. This will cover the primary end point, and 6 months after that for all of the people enrolled, since it is measured from the last person enrolled, not the first. After that the blinding is lifted. I assume this will lead to faster publication (especially of the primary end points), since they will not need to wait the full 24 months to start the data analysis.
First person given Ladarixin: https://www.dompe.com/en/media/press-releases/domp%C3%A9-announces-first-patient-enrolled-in-phase-3-trial-of-ladarixin-an-oral-investigational-cxcr1-2-inhibitor-in-new-onset-type-1-diabetes-t1d
US Clinical Trial: https://www.clinicaltrials.gov/ct2/show/NCT04628481
Europe Clinical Trial: https://www.clinicaltrialsregister.eu/ctr-search/trial/2020-001926-71/DE
Recruiting Locations
This study is recruiting from 58 different locations, all over the world and the United States. It is a large endeavor. I can not list them all here, but they are listed on the clinical trial record: https://www.clinicaltrials.gov/ct2/show/NCT04628481
If you are in Northern California, contact info is: Center of Excellence in Diabetes & Endocrinology (CEDE) , Sacramento, California, United States, 95821-2123, Contact: Gnanagurudasan Prakasam, MD prakasg@sutterhealth.org
United States locations include: Arizona, California, Colorado, Delaware, Florida, Georgia, Illinois, Indiana, Kansas, Kentucky, Massachusetts, New York, North Carolina, Pennsylvania, Texas, and Virginia.
International locations include: Belgium, Georgia, Germany, Israel, Italy, Serbia, and Slovenia (many sites in most countries).
Results From A Phase-II Study
The results of this study were published as a paper, here:
https://pubmed.ncbi.nlm.nih.gov/35589610/
and here are the key results:
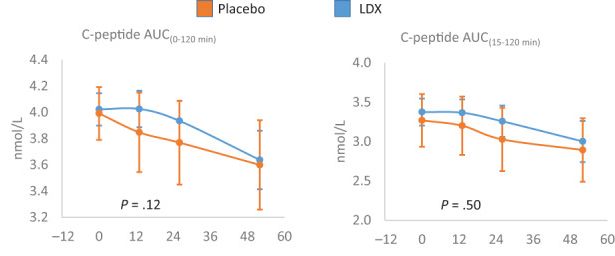 |
| Copyright 2022. Provided for educational purposes only. |
You can see that the people who got the drug (blue line) did better than the people who did not (brown or orange line). However, the P value is too high to be statistically significant (.12 versus .05 or lower). But also notice that even for the treated group, the value was never higher than it started. To me, that suggests that this drug might have stopped beta cell destruction for a few months, but never led to beta cell regeneration. This result has been seen in the past for other drugs, but - so far - has not led to effective treatments.
Clinical Trial Registration: https://www.clinicaltrials.gov/ct2/show/NCT02814838
The results of this study were published as a poster in the ADA 2020 conference: https://diabetesjournals.org/diabetes/article/69/Supplement_1/249-OR/56830/249-OR-A-Randomized-Double-Blind-Phase-2-Trial-of
Discussion
The big discussion point here is: why is Dompé running a phase-III study when their previous phase-II study was unsuccessful at the primary end point? There are three answers to that question and they are all important in their own way:
First, although the results were unsuccessful at 12 months, the same measurements were successful at 6 months. An optimist would say that the treatment worked, just not in the time frame that had previously been selected as the primary outcome. By giving the treatment for a longer period of time (12 months instead of 3), the researchers hope to have a successful outcome. In real life, the important time frame is not 6 months or 12 months, but a lifetime, so quibbling over exactly when a treatment is measured may not be productive.
Second, Dompé may know something that no one else knows, or they may be focusing on some specific results which they think is important to improving future results. Remember, they have been developing this drug for years. They also may have data from the earlier study which was not published, but which gives them hope not obvious to the rest of us. In fact, their ADA poster reporting on their previous results included this quote:
Although primary endpoint was not met, secondary and subgroup analyses suggest further investigation of LDX [Ladarixin] in new onset T1D may be warranted.
This quote certainly covers the 6 month results (which were a secondary result), but could be referring to other results which give Dompé more hope of success in the future.
Third, there is no requirement (legal, scientific, medical, or any other) that previous clinical trials must be successful in order to run more clinical trials on the same drug. As I'm fond of saying, in order to run the first clinical trial for a new drug, you need three things: a researcher who wants to run it, money to run it, and (in the US) a new drug approval from the FDA. However, the new drug approval only needs to show safety, not success of any kind. For a second clinical trial, you already have the new drug approval, so all you really need is a researcher and money.
In the past, this has lead to both good and bad results. In the case of Teplizumab, an early company chose bad end points, and had an unsuccessful clinical trial because of it. However, the researchers were able, years later, to get another company involved with different end points and have a successful trial. This led eventually to FDA approval. On the other hand, in the case of BCG, Faustman's lab ran unsuccessful trial after unsuccessful trial, using them as fundraising opportunities, but not (in my opinion) progress towards cure success.
My personal view is that Dompé's result is more like Teplizumab than like BCG. Also, giving the drug for longer seems like a direct way to convert the previous unsuccessful result to successful. So I'm optimistic about the overall chance of being successful in the primary outcome in the future. I'm much more curious as to the size of the result. It appeared that the untreated group's insulin production dropped (as expected during the honeymoon), but the treated group's insulin production stayed flat. Results similar to this have been seen before, but it remains a challenge to convert these types of results into a cure or a delay. Hopefully, this drug will do better.
Another interesting point about this study is that there is a secondary
end point, which is (to me) incredibly aggressive. If this end point
shows any response, it would be great news. It is "Percentage of
patients not requiring insulin therapy", which - in a certain sense - is
a measure of a practical cure. They are hoping that some people will not need
to inject insulin at all.
More To Read
Baylor participation: https://www.bcm.edu/healthcare/clinical-trials/h-49339
Phase-II Poster: https://diabetes.diabetesjournals.org/content/69/Supplement_1/249-OR
Phase-II Press Release: https://www.prnewswire.com/news-releases/dompe-presents-phase-ii-clinical-trial-results-for-ladarixin-in-new-onset-type-1-diabetes-at-the-american-diabetes-associations-scientific-sessions-301076774.html
No comments:
Post a Comment